A collaboration between the University of Utah and Sethera Therapeutics has discovered an enzyme which could unlock a new method of producing therapeutic peptides, expanding the druggable genome.
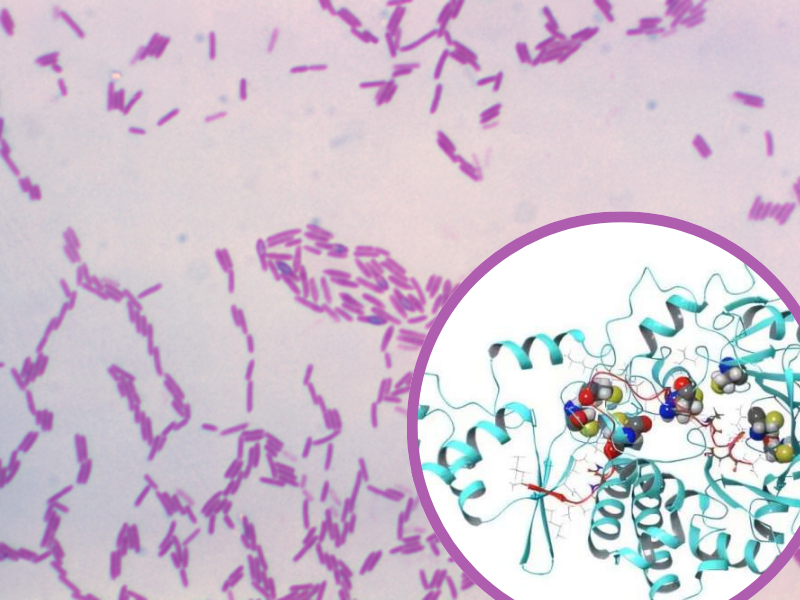
Their paper, published in Proceedings of the National Academy of Sciences, details how a naturally occurring enzyme found in the bacterium Paenibacillus polymyxa called PapB is able to conjoin two ends of linear peptide into macrocycles. These ring-shaped macrocycles are more stable and less easily broken down than their linear counterparts, making them well suited to therapeutic applications.
Lead author on the study, and CEO of Sethera Therapeutics, Karsten Eastman said: "Peptides that behave both like small molecules and biologics at the same time—that's the goal. This enzyme lets us program a durable thioether 'staple' across an unusually wide range of backbones in a single enzymatic step, massively expanding the design space we can test against difficult-to-hit biological targets.
The stabilisation of peptides, usually using disulfide bonds or other chemical methods, is a difficult problem in their therapeutic development. Bonds usually end up breaking down inside the body.
PapB offers an easier, cheaper and less time-consuming alternative to these methods, whereby the enzyme can be simply programmed to ‘staple’ peptides into macrocycles. This method widens the array of potential peptide medicines, opening up the door to increased cell penetration and oral peptide dosing, both widely sought after in the field.
Eastman added: "For discovery teams, that means faster iteration, richer and more diverse libraries, and scaffolds with the stability and permeability profiles needed to move from an intriguing hit to a viable therapeutic lead."
The authors of the paper have filed for a provisional patent (WO2023201372) for a modified PabB sequence for use as a drug development tool.