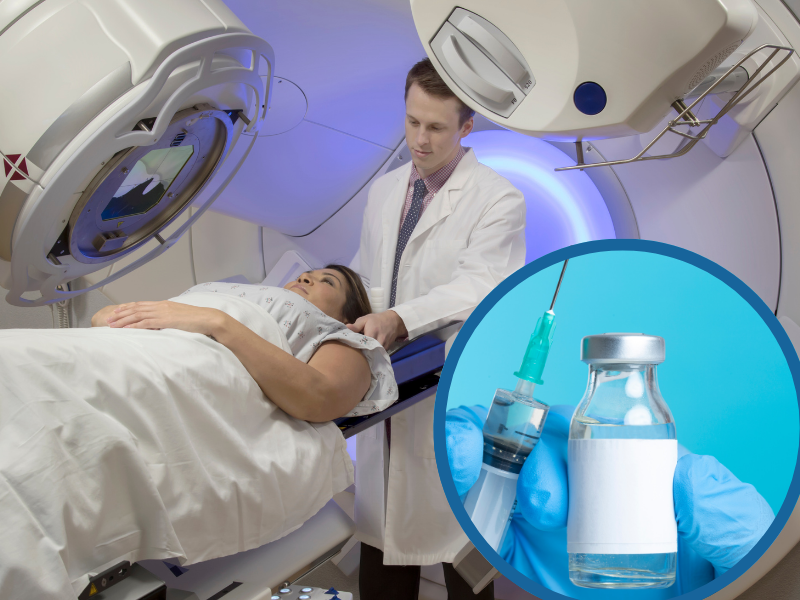
A new paper published in the journal Frontiers Immunology has reported positive results from a phase II clinical trial of a neoantigen peptide vaccine in combination with precision critical lesion extracorporeal radiotherapy (CLERT).
The open-label, multicentre phase II randomized study was conducted by researchers at the University of Hong Kong, Wenzhou Medical University and Case Western Reserve University, Ohio.
Although neoantigen peptide vaccines have shown some promise at treating solid tumours, in more advanced cases tumour burden and T cell function have made the modality less effective.
The study was dubbed iNATURE. It evaluated whether CLERT, a form of radiotherapy which involves delivering precise doses of radiation to specific, crucial areas within the tumor, can amplify the immune response triggered by the vaccine.
154 patients were enrolled at the University of Hong Kong-Shenzhen Hospital. Trial participants were males or females over the age of eighteen with advanced or recurrent malignancies diagnosed by pathology and imaging. Those patients had failed systemic standard therapy or disease progression prior to enrollment. The primary endpoint was progression free survival.
The combination therapy was used to treat patients with advanced malignant tumors who have progressed after systemic therapy. Patients received wither placebo with conventional treatment or the personalized neoantigen peptide vaccine and conventional treatment