Results published in Science Translational Archives, detail the successful application of a novel gene therapy for osteoarthritis. The phase I clinical trial was conducted by researchers at the Mayo Clinic and demonstrated safety and therapeutic effects of the novel gene therapy.
Christopher H. Evans, Director of the Musculoskeletal Gene Therapy Research lab at Mayo Clinic, said: "This could revolutionize the treatment of osteoarthritis."
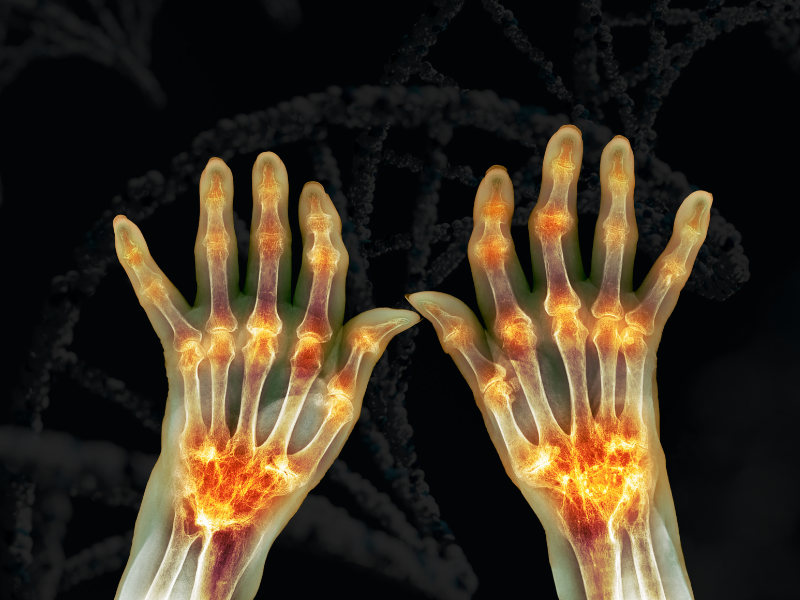
Osteoarthritis is a degenerative joint disease where the protective cartilage at the end of bones wears down over time. The disease can be painful, disabling, and is notoriously difficult to treat as medications injected into the joints tend to seep out of the target area.
Evans said: As far as I know, gene therapy is the only reasonable way to overcome this pharmacologic barrier, and it's a huge barrier."
Therefore, Evans’s lab has developed a gene therapy which induces cells develop anti-inflammatory molecules themselves. The molecule in question is IL-1 receptor agonist (IL-1Ra) which inhibits the pro-inflammatory cytokine IL-1: a primary driver of osteoarthritis symptoms.
The therapy delivers IL-1a to cells in the joints using an adeno associated viral vector (AAV). Preclinical testing in 2000 showed that the gene therapy was able to block the breakdown of protective cartilage. In 2015, they were granted investigational new drug (IND) approval.
Results from the trial showed that participants’ levels of IL-1Ra in the joint increased and remained elevated for at least a year. Furthermore, participants reported reduced pain and better joint function after treatment. No serious safety issues were observed.
“This study provides a highly promising, novel way to attack the disease,” said Evans.