Krystal Biotech has announced a label update, approved by the FDA, which expands the eligible patient population for its gene therapy beremagene geperpavec (B-VEC). B-VEC is the first gene replacement therapy for a non-cancerous skin disorder and also the first ever topical gene therapy.
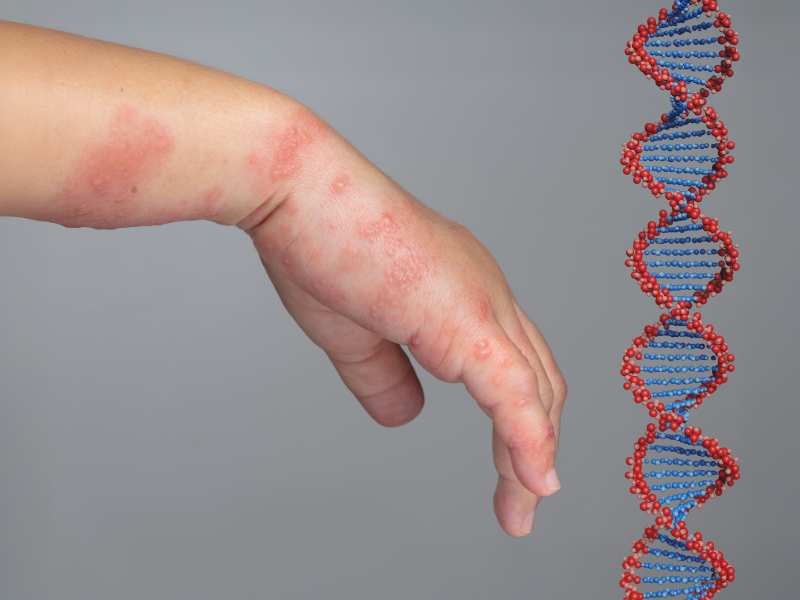
The gene therapy treats dystrophic epidermolysis bullosa (DEB), a rare disorder which is characterised by intense skin fragility leading to painful blisters. Now, the FDA has approved the use of the drug for paediatric patients from birth.
This update comes after B-VEC underwent an open-label extension trial in the US. Data from the trial collected after the therapy was launched showed high patient satisfaction after the treatment was applied by a caregiver or the patient themselves. Furthermore, no new safety concerns were reported.
“Enabling caretakers to apply VYJUVEK [B-VEC] during their standard of care regimen is an enormous positive change, allowing for increased convenience without sacrificing safety,” said Brett Kopelan, Executive Director of Debra of America, a charity focused on supporting epidermolysis bullosa patients.
“The Krystal team has always prioritized patient safety and convenience when it comes to the use of VYJUVEK and them advocating for these updates is not surprising given Krystal’s patient-centric approach. This aspect of the update to the label will only increase the quality of life of those living with this challenging disorder and that is exactly what our community needs.”
B-VEC is a non-invasive, topical gene therapy that delivers two COL7A1 gene copies to DEB wounds, enabling cells to produce normal COL7 proteins. It is approved in the US, Europe, and Japan, and is the first FDA-approved treatment for DEB.